Cancer Diagnosis Program (CDP) Melanoma Progression TMA
Design Overview
CDP Melanoma Progression Tissue Microarrays are designed to survey differences in biomarker prevalence in primary, recurrent and metastatic melanoma. This TMA set utilizes a cohort of 74 melanoma patients, each of whom is represented by one or more of the following discreet lesions: Primary tumor, Recurrent tumor, Lymph node metatastasis, Dermal metastasis, Distant metastasis, and Benign nevus. Multiple lesions are sampled from 46 subjects, 28 are represented by a single lesion, and the average number of lesions sampled per subject is 2.6. Normal skin controls from 25 additional subjects are also provided. The cohort is divided into to 2 case sets arrayed onto separate paraffin blocks. Each lesion and control is sampled in duplicate with a 0.6 mm core for a total of 390 melanoma tissue cores and 50 control cores spanning two TMA blocks. This array set also includes separate TMA containing HT-144, UACC903, and SK-Mel-2 melanoma cell lines and a test TMA with an assortment of 10 normal and malignant tissues sampled with 0.6 mm cores (2 subjects per tissue sampled in triplicate, 60 cores). The CDP melanoma progression TMAs were designed and constructed by a National Cancer Institute grantee, but their statistical power is not stated. No demographic or clinical annotations are available for this TMA set.
The table below gives the distribution of melanoma lesions and control samples across the TMA set.
TMA Maps
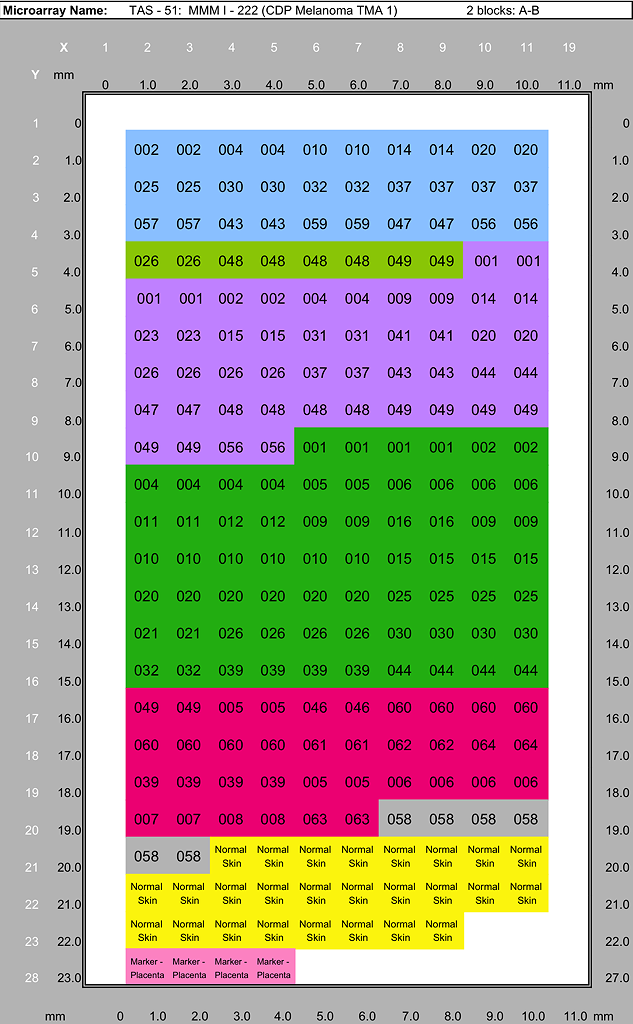
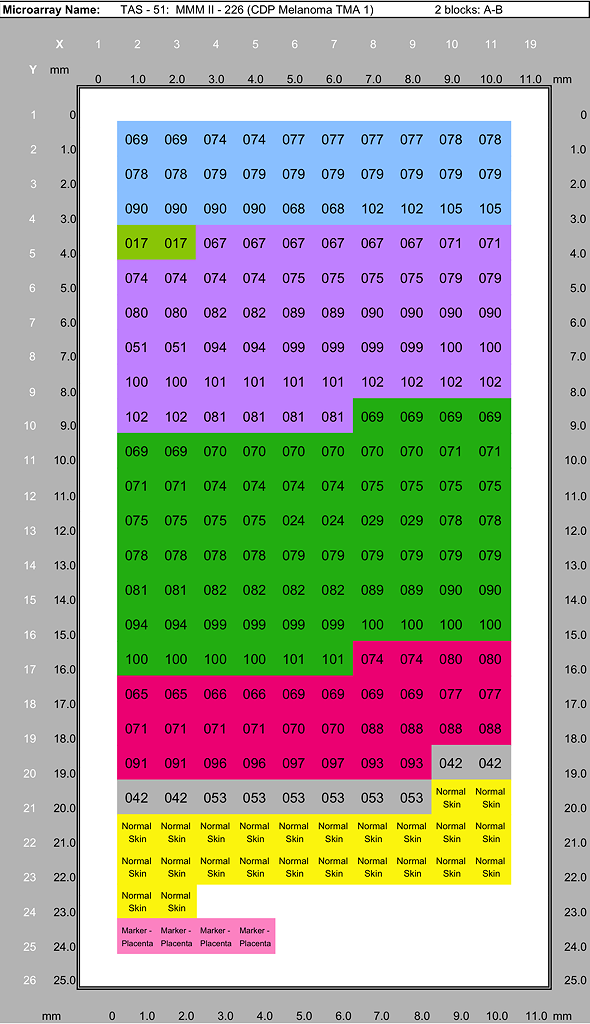
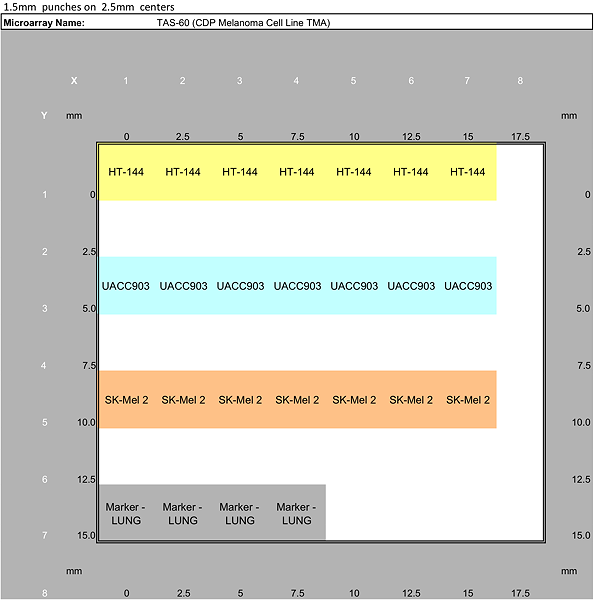

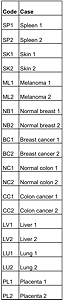
Melanoma Progression TMA Tissue List
Specimens represented in the CDP Melanoma Progression Tissue Microarrays case sets. Each case set is used to produce duplicate TMA blocks (A and B). A case set consisists of one section each of MMM I - 222 and MMM I - 226 plus one section each of TAS60 Cell Line Control, and one CDP Test TMA section.
| Type of Lesion | MMM I - 222 (CDP Melanoma TMA 1) | MMM II - 226 (CDP Melanoma TMA 2) | Total | |
|---|---|---|---|---|
| Primary Tumor cases | 15 | 15 | 30 | |
| Recurrent Tumor cases | 4 | 1 | 5 | |
| Lymph Node Metastatic cases | 23 | 27 | 50 | |
| Dermal Metastatic cases | 34 | 35 | 69 | |
| Distant Metastatic cases | 17 | 16 | 33 | |
| Benign Nevus cases | 3 | 5 | 8 | |
| Total melanoma tissue specimens | 96 | 99 | 195 | |
| Normal Skin control cases | 13 | 12 | 25 | |
| Total melanoma & control cores (each lesion/control sampled in duplicate) | 218 | 222 | 440 | |
|
TAS60 Cell Line TMA cores |
||||
|
HT-144 |
7 | |||
|
UACC903 |
7 | |||
|
SK-Mel2 |
7 | |||
|
Total cell line TMA cores |
21 | |||
|
CDP Melanoma Test TMA cases |
||||
|
Spleen |
2 | |||
|
Skin |
2 | |||
|
Melanoma |
2 | |||
|
Normal Breast |
2 | |||
|
Breast Cancer |
2 | |||
|
Normal Colon |
2 | |||
|
Colon Cancer |
2 | |||
|
Liver |
2 | |||
|
Lung |
2 | |||
|
Placenta |
2 | |||
|
Total Test TMA cores (each case sampled in triplicate) |
60 | |||
| These numbers represent the number of cases selected in the original design of the TMA. Due to the non-uniformity inherent to tissue samples and histologic techniques, not all cases will be represented in all of the TMA sections. | ||||
Array Maps, Annotation Keys and Datafiles
Below are downloadable files containing the complete annotation data for these TMAs. No additional data is available. Please contact the Mid-Atlantic Division with any questions.
- Download Full Annotation Data, & Maps (MS Excel)
